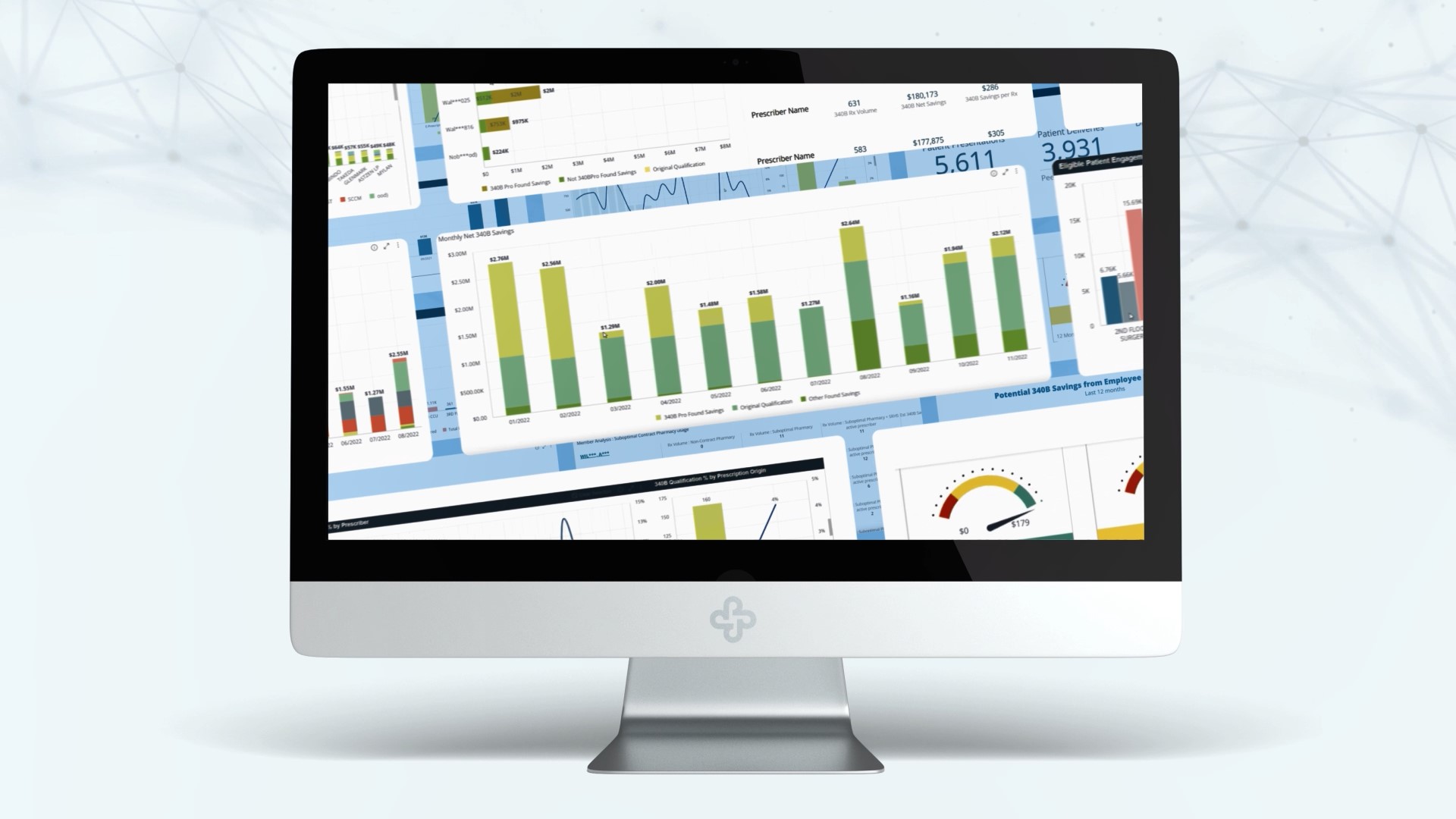
What we’ve learned. What it means for your 340B program. Between late 2019 and the end of 2023, ProxsysRx’s 340B Support program went from Zero to more than $500 million in savings generated for the hospitals and health systems we serve. In spite of the drug manufacturer restrictions that have devastated many hospitals’ programs, every … Read More
340B ESP
Enhancing Patient Care and System Revenue: The Case for Onsite Specialty Pharmacies in 340B Hospitals
Why a Specialty Pharmacy? (Overview) As chronic illnesses become increasingly common in the United States, the number of specialty medications for managing these conditions has skyrocketed. According to a report by The American Hospital Association, the number of Americans with chronic medical conditions will grow by a projected 9% between 2020 and 2030, an increase … Read More
Leveraging Partnerships With Drug Manufacturers To Overcome 340B Restrictions On Specialty Drugs
It’s no secret that the 340B Drug Pricing Program has been a lifeline for eligible health systems, enabling them to fill-in the massive revenue gaps inherent to the non-profit-hospital business model (and, in the process, support their mission of providing quality care to our nation’s most vulnerable patients) through access to discounted prescription refills. At … Read More
Drug Manufacturers’ 340B Restrictions Generate New Round Of Media Scrutiny & Criticism
In the past week, no less than three articles have called attention to the restrictions drug manufacturers have imposed to evade their legal responsibility to honor 340B program prescription discounts — as well as the impact those restrictions have had on 340B-eligible nonprofit entities’ ability to serve their patients and communities. “Don’t believe drug companies: … Read More
The 2023 Guide To Implementing An Effective 340B Program
The 340B-program landscape has changed dramatically. For the 340B program’s first 28 years after its 1992 inception, the basic list of Best Practices for eligible entities (the 340B basics, if you will) remained largely unchanged — aside from the dramatically-increased importance of using specialized software. But since the June, 2020 introduction of 340B ESP — … Read More
Why 340B Hospitals Should Now Build Their Own Specialty Pharmacies
340B ESP and drug manufacturer restrictions are only part of the reason As we noted in a previous post, for 340B-eligible hospitals dealing with increasingly squeezed bottom lines, an in-house specialty pharmacy offers enormous savings and revenue potential. Some covered entities generate as much as 600% in specialty pharmacy revenue from 340B drugs as they … Read More
Manufacturers Accelerate Pace And Severity Of 340B Drug Pricing Restrictions
There’s an old saying that it’s better to be rich and guilty than poor and innocent. It’s a sad truth that accurately reflects the current state of 340B manufacturer restrictions, particularly in the wake of the January 30, 2023 decision by the U.S. Court of Appeals for the Third Circuit — which was largely in … Read More
Brick Walls: Dealing With 340B Manufacturer Restrictions.
A ProxsysRx Team Member Speaks Out. I joined ProxsysRx in early 2021, and I’ve been devoted to 340B program management full-time since early 2022. I’m no stranger to heavily regulated programs. Nearly my whole professional career has been in some aspect of public health — preclinical, clinical and now post-clinical. I’m currently fully devoted to … Read More
The Ultimate Shame Of 340B ESP and Drug Manufacturer Restrictions
Making the rich richer at the expense of the poorest and most vulnerable. Why was the 340B program created? Nobody’s ever put it better than the Association of American Medical Colleges (AAMC): “Congress created the 340B Drug Pricing Program in 1992 to protect safety-net hospitals from escalating drug prices by allowing them to purchase outpatient … Read More
Seven Steps For Overcoming 340B ESP and Other Manufacturer Restrictions on 340B Pricing
ProxsysRx’s process for recovering lost 340B savings and revenue As we’ve noted in multiple earlier blog posts, 340B ESP is nothing short of a blatant, lawless tactic used by scores of drug manufacturers to disallow the discounts they are legally obligated to offer eligible entities. At the same time, a number of additional manufacturers are … Read More